
Peter Rost, M.D., is a former Pfizer Marketing Vice President providing services as a medical device and drug expert witness and pharmaceutical marketing expert. Judge Sanders: "The court agrees with defendants' view that Dr. Rost is a very adept and seasoned expert witness." He is also the author of Emergency Surgery, The Whistleblower and Killer Drug. You can reach him on rostpeter (insert symbol) hotmail.com. Follow on https://twitter.com/peterrost
Saturday, June 30, 2007
Friday, June 29, 2007
BrandWeek ruffles some feathers . . .
Which makes this story even more funny: The 'J&J Girl Scout Drug Dealer' Thing -- a Lesson in How Media Viruses Work
MedAdNews "Pharma Blogs: Week in Review"



Click here to subscribe to Pharma Blogs: Week in Review or our other eNewsletter.
Did Astra-Zeneca illegally market Seroquel for off-label use?
 The state of Pennsylvania thinks so. In February 2007, Pennsylvania filed a lawsuit against AstraZeneca, and other atypical makers, alleging the drug giant illegally marketed Seroquel off-label for unapproved uses.
The state of Pennsylvania thinks so. In February 2007, Pennsylvania filed a lawsuit against AstraZeneca, and other atypical makers, alleging the drug giant illegally marketed Seroquel off-label for unapproved uses.Seroquel is only FDA approved to treat acute manic episodes associated with bipolar I disorder and schizophrenia, and yet it is one of the most widely prescribed drugs in the world.
And I'm not sure what's going on at AstraZeneca, but this company generates more whistleblowers than anyone else.
So someone thought a few documents might be interesting in light of the allegations above.
The documents below is what AstraZeneca is using to educate doctors about areas Seroquel is not approved for.
Click on them to read and view full size.

But there's more. Seroquel was approved for bipolar depression only very recently, in October 2006.
So what did AstraZeneca do? Well, they made sure doctors were well educated about this area six months before they got the indication. See the date on the business reply card below (click to enlarge).

Did any of this violate anything?
I guess the state of Pennsylvania may think so, but we'll see . . .
Blog post of the day.
Jun 29th, 2007 by impactiviti
Time was, corporate misdeeds could be pretty effectively covered up. Sure, some scandals would leak out and make headlines, but information could often be contained, insiders constrained, or ignorance feigned.
No more. We’re in a time of new transparency.
It’s getting very difficult to keep the lid on wrongdoing, and I, for one, think that is a good thing. What has changed? In a word, electronic communications.
Damning information is leaking out all over nowadays. In our industry, there has been a significant uptick in corporate e-mails, Powerpoint slides, documents, and other documentation of wrongdoing flowing out and becoming public. Much of this has occurred through the emerging pharma blogger community (most notably, Peter Rost over at Question Authority; but now others, such as Ed Silverman at Pharmalot, are being given such info).
Of course, a lot of “after the fact” electronic information gets exposed during trials, as archived e-mails and other documents are unearthed. But now, we no longer have to wait for the discovery process of trials. We’ve seen information leaked, exposure occur, investigations begun, and people fired within weeks - because blogs suddenly make it possible to publicize information almost immediately. Including pictures and videos (far more ubiquitous nowadays).
This has its dangers, of course. Most bloggers are not trained journalists. Sometimes due diligence has not occurred. Rushes to judgment can occur. But when clear electronic evidence is brought to light…well, a refusal to take action suddenly becomes less of an option.
This forced transparency can have a salutary effect. Ethical behavior may be forced on those who otherwise would blur the lines. If you have nothing to hide, you have nothing to fear. But if you’re playing games, the likelihood now is far greater that you’ll pay the price. Very publicly.
Thursday, June 28, 2007
Pfizer's HIV division hit by a supersonic boom.
 First Pfizer had to deal with the maraviroc scandal and delayed FDA approval.
First Pfizer had to deal with the maraviroc scandal and delayed FDA approval.Now Ed Silverman is taking a second stab at this amazing story with juicy new revelations about how Pfizer's HIV sales force marketed Viracept.
If you are a visitor from the DoJ or FDA you really don't want to miss the second installment of this story, which includes lots of e-mails and names:
Go here.
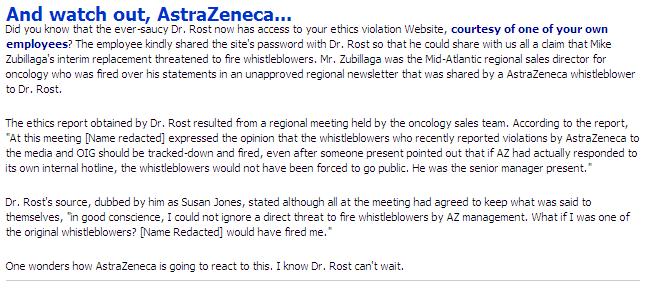
AstraZeneca ethics report claims that Zubillaga's interim replacement threatened to fire whistleblowers . . .
 I have just received access to the report number and pass code for a recent report on AstraZeneca's ethics violation web site, http://www.azethics.com/. (Click on images to enlarge.)
I have just received access to the report number and pass code for a recent report on AstraZeneca's ethics violation web site, http://www.azethics.com/. (Click on images to enlarge.)Report Submission Date
6/18/2007
Reported Company/Branch Information
Name: USA
Location: Mid-Atlantic Oncology Regional Meeting
City/State/Zip: Baltimore, MD, USA
Violation Information
Violation Type
Retaliation
What is your relationship to AstraZeneca?
Employee
Please identify the person(s) engaged in this behavior:
[Name redacted] - Northeast Oncology Regional Sales Director
Do you suspect or know that a supervisor or management is involved?
Yes
If yes, then who?
[Name redacted] - Northeast Oncology Regional Sales Director
Is management aware of this problem?
Do Not Know / Do Not Wish To Disclose
What is the general nature of this matter?
Threats of retaliation against whistleblowers.
Where did this incident or violation occur?
Baltimore, MD
Please provide the specific or approximate time this incident occurred:
June 13th, 2007
How long do you think this problem has been going on?
1 to 3 months
How did you become aware of this violation?
I observed it
Details
June 13th 2007
This took place today at a regional meeting in Baltimore MD. Present were oncology pharmaceutical reps and their district managers from the Mid-Atlantic oncology region.
[Name redacted] is the Northeast Regional Sales Director for oncology. He also served as the interim Mid-Atlantic Regional Sales Director after Zubillaga was fired.
At this meeting [Name redacted] expressed the opinion that the whistleblowers who recently reported violations by AstraZeneca to the media and OIG should be tracked-down and fired, even after someone present pointed out that if AZ had actually responded to its own internal hotline, the whistleblowers would not have been forced to go public. He was the senior manager present.
According to AstraZeneca's Corporate Integrity Agreement with OIG "Consistent with the open compliance environment that AstraZeneca strives to maintain, company policy prohibits retaliation or retribution of any kind following the good faith reporting of a suspected violation."
Follow-Up Questions/Comments
The company has submitted a comment or a question for you to answer.
6/19/2007 8:48 AM - AstraZeneca takes allegations of wrongdoing very seriously. This matter has been sent for further investigation. AstraZeneca will investigate this matter promptly, thoroughly and diligently and will takes appropriate steps based on the outcome of the investigation.
Follow-Up Notes
Jun 27, 2007, 9:56 AM
[Name redacted] had each of us vow that anything said at this meeting would stay secret.
FYI - The premise of this meeting was to 'move forward', after numerous recent scandals, by forming a committee of representatives to make suggestions to management.
Follow-Up Questions/Comments
There are no questions asked or comments posted by the company.
Chat Transcripts
There are no chat transcripts for this incident.
Read AstraZeneca's official policy on retaliation, click on image below!

My new source inside AstraZeneca, "Susan Jones," wrote the following when she sent the report code and password to me:
"Dr. Rost, the premise of our meeting was to form a group of AZ oncology representatives to 'move forward' after the recent scandals in the region. We all agreed to keep what was said at the meeting to ourselves. However, in good conscience, I could not ignore a direct threat to fire whistleblowers by AZ management. What if I was one of the original whistleblowers? [Name Redacted] would have fired me. I picked up your book after the 'bucket of money' scandal and was inspired enough to override my fear and contact you. "
"Drugwonks Needs a Proofreader"
Wednesday, June 27, 2007
"The Sludge Report: More Mewlings From Peter Rost"
The Sludge Report: More Mewlings From Peter Rost
Robert Goldberg
Lest anyone be uncertain that Peter Rost is not a small-minded person who blogs without regard to the totality of the facts, here is the context of Rost's gleeful pursuit of Pfizer's renewed effort to market Viracept, an "old drug" as the consistently vindictive and inaccurate Rost calls the HIV medicine.
Story continues here.
Oh, and by the way, apparently the working name for this story, based on the link, was actually The Smallminded Peter Rost and his band of Munchkins (I'm really sorry they didn't use that title, because it is pretty funny): http://drugwonks.com/2007/06/the_smallminded_peter_rost_and_his_band_of_munchkins.html
Here's the part about the story above which is even more funny; the fact that Robert gives me credit for the story:
I didn't write it.
Ed Silverman did. I just reproduced what he'd written. But I guess it isn't as much fun for Big Pharma to bang on Ed as it is to kick my head.
Secret CIA document reveals cooperation with drug industry.
Pfizer shuts down maraviroc training and launch meeting.
Background on maraviroc story.
More bad HIV news for Pfizer
U.S. regulators will review Isentress, an investigational drug for HIV infection, on a priority basis, and a decision is anticipated by mid-October, Merck & Co. said on Wednesday.
Reuters has the story.
CIA's release of documents implicates drug industry!
But there was one section New York Time missed, which New Scientist picked up, and this excellent magazine writes:
"Go to page 416, and you will learn of a behavioural drug screened as part of "larger programme, in which the Agency had relations with commercial drug manufacturers, whereby they passed on drugs rejected because of unfavorable side effects". Drugs deemed interesting were later tested on "volunteer members of the Armed forces". The programme was apparently considered "defensive, in the sense that we would recognize certain behavior if similar materials were used against Americans".
It's the complicity of the pharmaceutical industry, passing on drugs known to be harmful, that I find most disturbing. Any ex-spooks or pharma executives care to comment?"
NJ Star-Ledger: "Pfizer probes marketing complaint"
I guess the others are too afraid they'll lose advertising revenue if they touch this story.
Follow a whistleblower case live!
But the whistleblower, whom we'll call Mary Smith, just contacted me and asked me to hold off. She thought we might get more mileage out of this if I simply followed the story quietly, and then report back to you.
The beauty is, of course, that AstraZeneca has no idea which one of all the complaints they get it is that I'm monitoring. So they have to treat everyone right.
Just to make things even more interesting I also have another AstraZeneca story brewing, where the same request has been made.
I guess what all of this proves is that if employees thought the companies would do the right thing, they'd never contact me or anyone else. And they still have their hopes up, asking me to stay silent unless the company shows bad faith.
Interview with former Pfizer finance executive and whistleblower Ashok Idnani.
A Pfizer insider appears to have tried to use the article to discredit the whistleblower,Ashok Idnani, leaving comments both on CafePharma and on this blog.
On CafePharma this comment appeared, "Looks like Rost got burned on his India stories. The plant that Pfizer supposedly sold for 10% of it's value was actually sold at slightly higher than the market rates. I wonder if that India stuff was a joke someone played on Rost. It is pretty funny though, mainly because of how much time he spent on it. Hopefully he can check out his stories in the future so he does not get duped again."
And on my blog, this comment was posted, "Well, according to the article posted on this website the property was sold for higher than market rates. According to the Indian contact it was sold for one-tenth the value. So, it looks like that aspect was a non-issue. I am not sure what the guys credentials are, but maybe Dr. Rost can post them too. Most companies conduct annual written reviews so that would be a good thing to publish in order to tell how well credentialed this guy was. Maybe the other allegations have a basis but I would think that most rational people might have a legitimate reason to question them. I mean, every company hires detectives and it is not always for a nefarious reason. For all I know it the people being followed could have threatened to blow up a plant. The Indian contact has a credibility problem right now, if he could establish that he was receiving good reviews then the general public would be more likely to believe he was retaliated against. Maybe he was just a crazy guy with an ax do grind and this was his best forum."
So, I thought, why not let the whistleblower reply?
Mr. Idnani's what do you think about these comments which question you credentials?
This appears to be written by a Pfizer insider. I can reply to that easily sending you my last appraisals done in 2001 and 2002 which were good, including an email to me in Jan 2002 from then country manager Hocine appreciating my role in integration activities of Pfizer-Parke Davis. Starting January 2003 they "froze" my salary and bonuses and there were no appraisals since then.
But what about the allegation that the article disproves that the plant was sold for less than its real value?
As for the property being sold for more than market value . . . this is simply not true. The government valuation of the property of Rs. 1800 million (conservative) has not changed. Only the Stamp Duty demand has been waived. The charge remains that Pfizer India sold the property at 10% of potential market value, and that is no doubt an undervaluation.
So aren't you just an employee with an ax to grind?
As for the comment about "an ax to grind" in your forum, the facts speak for themselves. I had approached my superiors in 2002, 2003, 2004 and ultimately Pfizer Inc & Jeff Kindler in November 2005 and until today the outcome of the November 2005 investigation has not been disclosed to me.
Is anything wrong in the Business World story?
Pfizer India has only now, falsely, started telling the newspapers "he has been told, his charges are baseless." So only after the postings on your blog and without any communication to me from Pfizer NY, who investigated the charges, and from whom I had been demanding the outcome and the detailed investigation reports, which was denied to me, do they say this.
So what about this Pfizer apologist who claims “every company hires detectives and it is not always for a nefarious reason"?
No company should authorize breaking into your bank accounts and start tracing your telephone calls, this is illegal. And bribing government officials is illegal. If you do that (and it was done by Pfizer with knowledge of Jeff Kindler, since I notified him) then you should also be sacked, just like HP's chairwoman was forced out. But instead the salary of the current Pfizer-India country manager was increased by a phenomenal 111%.
So do you think these comments were written by a Pfizer PR hack?
Where is the credibility problem really - with the person accusing or the accused? You have the documents and the facts. Let the people who read this decide!
Tuesday, June 26, 2007
"Five Facts PhRMA Will Ignore About Rx Importation"
Emanuel, Emerson, DeLauro, Berry Call for Passage of Importation Legislation
WASHINGTON, D.C. — In advance of today’s PhRMA’s staff “briefing” on prescription drug importation, U.S. Representatives Rahm Emanuel (D-IL), Jo Ann Emerson (R-MO), Rosa DeLauro (D-CT), and Marion Berry (D-AR) released the top five facts PhRMA won’t tell you in today’s session:
#5 The same brand name drugs cost 35-55% percent less in other countries than they do in the United States. - The Congressional Budget Office
#4 Drug importation has been in place in the European Union for more than 20 years with no safety problems. – Dr. Peter Rost, Former Vice President of Marketing, Pfizer
#3 The pharmaceutical industry has imported drugs and sold them in the U.S. for decades. In fact, 40 percent of the drugs consumed by Americans today are made in foreign manufacturing plants. – Congress Daily, June 5, 2007
#2 Prescription drug importation will result in $50 billion in direct savings alone over the next decade, a $10 billion benefit to the federal budget. – The Congressional Budget Office
#1 Passage of the Pharmaceutical Market Access and Drug Safety Act will finally assure the security of our drug supply.
Quote of the day II: "Now Viracept is being investigated"
--------------------------------------------------------------------------------
#1
Today, 09:22 AM
Anonymous
Posts: n/a
Now Viracept is being investigated
--------------------------------------------------------------------------------
who is the sales rep feeding this stuff?
http://www.pharmalot.com/2007/06/at-pfizer-improper-marketing-is-an-infectious-disease-drugmaker-probes-another-aids-med/
--------------------------------------------------------------------------------
#2
Today, 01:10 PM
Anonymous
Posts: n/a
Re: Now Viracept is being investigated
--------------------------------------------------------------------------------
There are numerous people like this one that are ready to bring Pfizer down based on the way the way we were treated through Ats. I am one of them. A tenured very successful Pfizer rep that will never forget how I was treated. I think uncle Jeff underestimated our resolve and why some of us were so successful. We will continue to provide the goods to bury the devil.
A sovereign nation sues Pfizer. $9 billion at stake on July 20.
In 1997 Trovan was approved by the FDA to treat certain infections, but not on children, and not for epidemic meningitis. After two years on the market hundreds of reports found the drug to cause liver toxicity and it is no longer sold.
More info here.
Quote of the day: "The only way to meet our goal is to sell off-label."
The HIV sales force scandal just exploded in Pfizer's face.
Ed Silverman over at Pharmalot just followed up with his own Pfizer HIV sales force whistleblower story, complete with e-mail and power point presentation, focusing on Viracept, which is the drug this HIV sales force is actually selling.
This well researched story is a must read and I'm simply reproducing Ed's outstanding work below. I can't wait for his next installment!
At Pfizer, Improper Marketing Is An Infectious Disease; Drugmaker Probes Another AIDS Med
June 26th, 2007 8:58 am By Ed Silverman
 Over the past few weeks, Pfizer has had nothing but trouble with its new AIDS drug, maraviroc. The FDA unexpectedly delayed approval and the reasons aren’t clear. Meanwhile, the drugmaker quietly began investigating allegations that its sales team was instructed to promote maraviroc to physicians. This is a big no-no, because the drug still isn’t approved and this would violate a Corporate Integrity Agreement with the feds.
Over the past few weeks, Pfizer has had nothing but trouble with its new AIDS drug, maraviroc. The FDA unexpectedly delayed approval and the reasons aren’t clear. Meanwhile, the drugmaker quietly began investigating allegations that its sales team was instructed to promote maraviroc to physicians. This is a big no-no, because the drug still isn’t approved and this would violate a Corporate Integrity Agreement with the feds.
As it turns out, Pfizer is also investigating charges that the same HIV sales force was encouraged to improperly promote Viracept, an aging AIDS drug. Sales reps were given unapproved training and detailing materials (see below). The allegations, which also involve unapproved funding of CME programs, were made by a sales rep who now complains of retaliation. A Pfizer spokesman confirms the investigation is under way, adding that “we take any concerns about any appropriateness of activities involving the company very seriously.”
Why did this happen? A mix of panic and frustration. Pfizer got into the AIDS business in 2000 by acquiring Warner-Lambert, which itself had bought Agouron Pharma and its Viracept, or nelfinivir, a new AIDS med that generated about $420 million in sales in 1998. But Viracept was soon eclipsed by other drugs, such as Kaletra, Sustiva and Reyataz, and by last year, annual sales dwindled. Underscoring the point, Viracept is no longer listed separately in Pfizer financial reports. (Look here for the decline. On the far left, Viracept is the green line that begins at 20 percent and later plummets).
“We call it has-been-avir, not nelfinivir” says the sales rep. “They had to do something. And so we were given all sorts of materials to boost business. You know, a sales rep just doesn’t wake up one morning and say ‘I’ll use these unapproved studies or slides or whatever.’ It has to come from somewhere. And we were told that our performance was based on whether we were able to use that material in the field. But it violates Pfizer’s own policy. That’s why I reported these things.”
 Here’s one example. This e-mail was written by a Pfizer professional science liasion, a position more commonly called medical science liasion at other big drugmakers. The note encourages HIV sales reps to show doctors a set of slides entitled ‘Treatment Considerations for HIV Today and Tomorrow.’ But the sales rep points out that the slides disclose a problem.
Here’s one example. This e-mail was written by a Pfizer professional science liasion, a position more commonly called medical science liasion at other big drugmakers. The note encourages HIV sales reps to show doctors a set of slides entitled ‘Treatment Considerations for HIV Today and Tomorrow.’ But the sales rep points out that the slides disclose a problem.
From: REDACTED
> > Sent: Thursday, November 09, 2006 11:09 AM
> > To: Wilson, Blythe Ashley; Weiss, Lawrence; Wynn, Jeffrey S;
> > Bilawsky, Leslie J; Miller, Vanessa Z; Raymond, James; Fazzina,
> > Douglas; DeRamus, Lisa A; Turner, Edward; Shimp, Christine Lynn
> > Cc: Zaleski, Carolyn
> > Subject: Slides - Today’s HIV Patient
> >
> > New York/New England Team,
> >
> > Attached are the slides that we discussed at the POA. This is an
> > excellent slide deck to present to your providers to provide value,
> > and sell Viracept. This is for your information, and to be shared
> > only with your providers. Please let me know if you have any
> > questions on utilizing these slides in your territory.
> >
POA is plan of action, a scheduled meeting for a sales force to discuss strategy. You can either flip through the slide set or go right to page 19, and there’s a chart that compares Viracept with other protease inhibitors and their affect on lipid levels. However, this is a prospective, observational study, not a head-to-head trial. In other words, it’s an inappropriate comparison.
This would appear to violate Pfizer’s own policy, which is described in The Field Guide, a manual given sales reps: “A comparative claim generally must be backed up by at least two adequate, well-controlled studies in which the drugs were compared head-to-head using comparable dosage regiments or a single, large, well-controlled study.” See pgs. 21 and 22.
The sales rep also alleges the slides and other materials were never approved by the Pfizer Review Committee, a mandatory step. This is the sort of activity that Pfizer is required to report to the HHS Office of Inspector General as part of its CIA, which Pfizer signed as a result of its settlement over improper marketing of Neurontin. “…Our obligations under these settlements include…disclosing activities by Pfizer colleagues that are non-compliant with the health care laws,” according to the drugmaker’s own rules on page 9 in The Field Guide.
The Viracept investigation is occuring at the same time as the maravoric investigation, since the same marketing and sales teams were involved and many of the same sales reps are being questioned. The probe is being handled by the same outside lawyer, Ropes & Gray’s Josh Levy, who spoke with the sales rep who provided this information. The maravoric investigation was first disclosed in April by Peter Rost, who has subsequently posted numerous updates on the way Pfizer has handled the problem.
The slide set was just one of many materials and documents the sales rep tells us were part of a so-called road map designed to make it possible for the HIV sales force to ultimately convince doctors to write Viracept scrips. Internally, this effort was known as ‘promotional mapping,’ which the sales rep says contained various clinical arguments that also came in the form of obscure studies.
 “This was a way to put all of these unapproved materials into one place and then train us. But it didn’t just appear – the sales rep didn’t get together one Sunday afternoon by themselves and decide to use these materials. We weren’t renegade reps. We went to a POA and it was represented to us as a way to do business.”
“This was a way to put all of these unapproved materials into one place and then train us. But it didn’t just appear – the sales rep didn’t get together one Sunday afternoon by themselves and decide to use these materials. We weren’t renegade reps. We went to a POA and it was represented to us as a way to do business.”
Two notes to Pharmalot viewers:
1 – There will be a few additional installments in coming days.
2 – The sales rep was granted anonymity due to this person’s role as a whistleblower.
Monday, June 25, 2007
I love it!
The Whistleblower And The Drugmaker…… Tomorrow Morning In Pharmalot
I can't wait to read the story. And if we can get this going with various whistleblowers coming forward on various blogs, then I can eventually retire and just enjoy the reading!
Pfizer's lips are zipped . . .

. . . when it comes to the finance scandal Question Authority revealed in India . . . according to Business World.
Pfizer has apparently issued to "gag order" to Pfizer India.
Story here.
Pfizer's India Scandal Raises Questions in Mainstream Media
May 16, 2007: Pfizer Finance Executive Blows the Whistle - Part One
May 17, 2007: Pfizer Finance Executive Blows the Whistle – Part Two
May 18, 2007: Pfizer Finance Executive Blows the Whistle – Part Three
Click on images below to read full size article:


Sunday, June 24, 2007
Rost caught in the chemo treatment area with AstraZeneca gifts!
Saturday, June 23, 2007
Midsummer Celebration New York 2007
Well, you can always come next year and participate in this hedonic tradition when men put flowers in their hair and tall, blonde women dance around a May pole.
(Click on images for large size.)



















Friday, June 22, 2007
Huh???
As to what Robert Goldberg is trying to say, that is a bit hard to figure out. But I think he is really, really, upset the FDA didn't approve Pfizer's maraviroc. What do you think?
State of Fear at the FDA
Robert Goldberg
What's up with the FDA issuing an approvable letter on maraviroc? Well according to Peter Rost (who should know since he is the master of manipulating golden parachutes for himself. Rost's next book: How To Collect Fat Paychecks From ShortHills By Suing Those You Work For) it was to allow outgoing Pfizer R and D execs to short their options. Yeah right. And all those imported drugs made in China ARE safe, just like the toothpaste and petfood.
Anyways, if you were an FDA drug reviewer you now have two choices: become a snitch for Senator Grassley or Congressman Waxman or simply wait till the very last minute to approve a drug. You never know when -- in the words of the owner of the Steven E. Nissen Healthy Heart Fund -- you might be approving another 9=11.
Maraviroc was a targeted therapy for people facing multiple drug resistant HIV...but hey, what's a few months while the FDA sorts out an even lower incidence of liver problems than Tylenol? We now live in a country where in some parts you have to be 19 to buy cold medicines with dextromethorphan. That's right folks, you can fight for your country but you can't buy Robitussin.
The infantilization of American continues apace.
Midsummer's Eve is Here!
 I'm off to celebrate the Swedish Midsummer's Eve at 5 - 8 pm in Robert F. Wagner, Jr. Park, Battery Park City in New York. Come by if you want to! This is an old Viking tradition that harks back to Sweden's hedonic past. But don't worry, the whistleblower stories for next week are ready to go!
I'm off to celebrate the Swedish Midsummer's Eve at 5 - 8 pm in Robert F. Wagner, Jr. Park, Battery Park City in New York. Come by if you want to! This is an old Viking tradition that harks back to Sweden's hedonic past. But don't worry, the whistleblower stories for next week are ready to go!This is arguably the most important holiday of the year in Sweden, and one of the most uniquely Swedish in the way it is celebrated, even if it has been influenced by other countries long ago.
The main celebrations take place on the Friday, and the traditional events include raising and dancing around a huge maypole. One typical dance is the frog dance. Before the maypole is raised, greens and flowers are collected and used to cover the entire pole.
 Raising and dancing around a maypole (majstången or midsommarstången) is an activity that attracts families and many others. People dancing around the pole listen to traditional music and many wear traditional folk costumes. The year's first potatoes, pickled herring, sour cream, and possibly the first strawberries of the season are on the menu. Drinking songs are also important at this feast, and many drink heavily.
Raising and dancing around a maypole (majstången or midsommarstången) is an activity that attracts families and many others. People dancing around the pole listen to traditional music and many wear traditional folk costumes. The year's first potatoes, pickled herring, sour cream, and possibly the first strawberries of the season are on the menu. Drinking songs are also important at this feast, and many drink heavily.Read more here.
Dr. John LaMattina: The Lucky PFE Executive

This week, based on the maraviroc fiasco and the failure to develope the Coley cancer drug Pfizer stock dropped like a rock.

Congratulations Dr. LaMattina!
MedAdNews "Pharma Blogs: Week in Review"



Click here to subscribe to Pharma Blogs: Week in Review or our other eNewsletter.
Thank you for birthday gifts, Astra-Zeneca!
 Astra-Zeneca apparently noticed that I celebrated my birthday a few days ago with a little song.
Astra-Zeneca apparently noticed that I celebrated my birthday a few days ago with a little song.So yesterday I got this wonderful box with all kinds of goodies from Astra-Zeneca:
Pens, notepads, tumor measuring device, bags, hand gel . . . thank you so much, Astra-Zeneca!





