And now that launch has been delayed.
Perhaps it isn't surprising that the FDA took a keen interest in the maraviroc whistleblower stories on this blog before making the decision to not approve maraviroc on June 20. And even less surprising if the whole situation gives Pfizer ants in their pants.
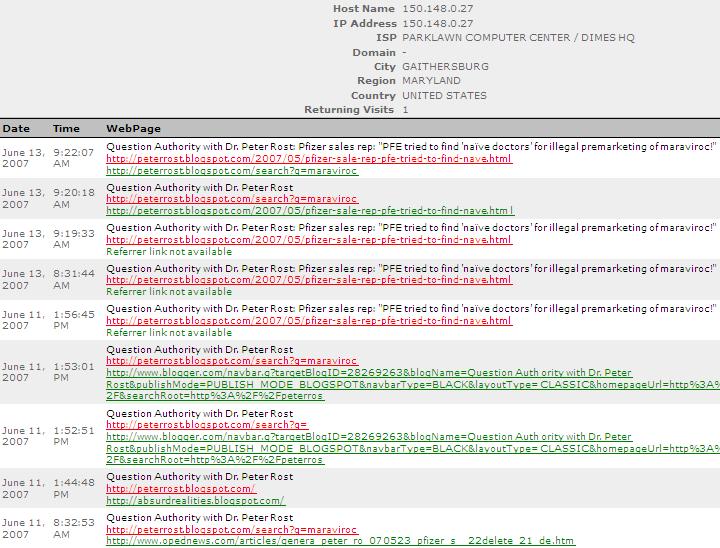
Parklawn Computer Center is the label for FDA's IP address, (confirm here).
You can find the most recent maraviroc whistleblower stories here.
No comments:
Post a Comment