Peter Rost, M.D., is a former Pfizer Marketing Vice President providing services as a medical device and drug expert witness and pharmaceutical marketing expert. Judge Sanders: "The court agrees with defendants' view that Dr. Rost is a very adept and seasoned expert witness." He is also the author of Emergency Surgery, The Whistleblower and Killer Drug. You can reach him on rostpeter (insert symbol) hotmail.com. Follow on https://twitter.com/peterrost
Showing posts with label Monogram Biosciences. Show all posts
Showing posts with label Monogram Biosciences. Show all posts
Thursday, June 21, 2007
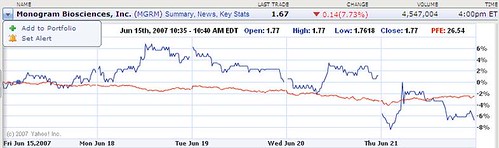
Monogram Biosciences stock: Panic selling after news of delayed Pfizer HIV drug
Perhaps the initial panic selling of Monogram Biosciences stock this morning shouldn't be surprising. Monogram manufactures a diagnostic test used to identify HIV patients who would respond to Pfizer Inc's maraviroc drug, which didn't get the expected FDA approval on June 20.


Tuesday, June 19, 2007
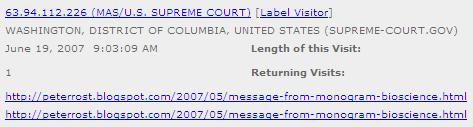
The U.S. Supreme Court Visits Question Authority
Thursday, May 24, 2007
Monogram Bioscience investors had early warning.
 Many Monogram Bioscience investor were mighty upset when I first revealed the maraviroc allegations on April 30.
Many Monogram Bioscience investor were mighty upset when I first revealed the maraviroc allegations on April 30.That isn't surprising.
While maraviroc is too small to move Pfizer stock, Monogram Bioscience depends on the launch of maraviroc, so they can sell a blood test to determine if a patient falls into the category that should be treated with the Pfizer drug.
Almost a month has now passed with more revelations.
So what has happened with Monogram Bioscience stock?
Well, investors who sold after my first revelation have saved a bundle.
The stock has since experienced heavy selling pressure and dropped sharply on increasing volume.
Investors who didn't sell have so far lost about 20% of their money in less than a month.
Uh-oh.
The market has spoken.
And while a technical chart analysis would indicate that the stock is getting ready for an upside move, no one knows what will be revealed next week.
That's the downside with technical analysis.
Wednesday, May 02, 2007
FDA and NY State Attorney General Review Pfizer HIV Allegations
My story "Pfizer whistleblower accuses company of using sales force to illegally market new AIDS-drug before FDA approval" has received the attention of both the FDA and the New York State Office of the Attorney General.
How do I know this?
They came by yesterday, reading all they could. Uh-oh.
Below are just a couple of samples.
NYS Office of the Attorney General came from--CafePharma (!)--and a post about my story:
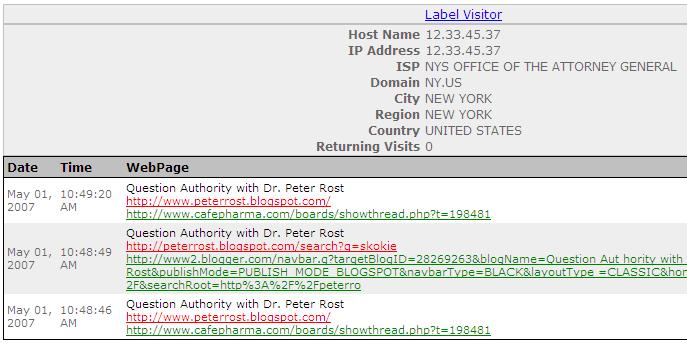
Parklawn Computer Center is a US Government Computer Center for the Federal Food and Drug Administration. Verify here.
How do I know this?
They came by yesterday, reading all they could. Uh-oh.
Below are just a couple of samples.
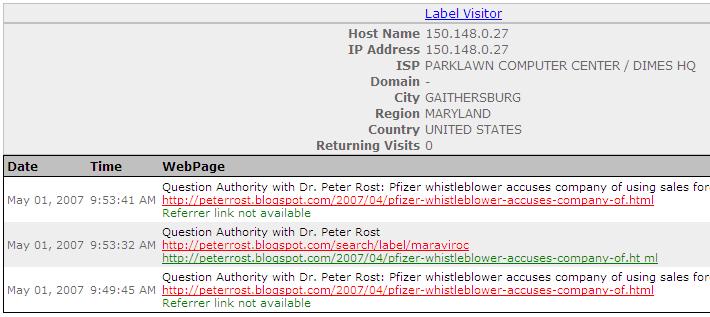
NYS Office of the Attorney General came from--CafePharma (!)--and a post about my story:

Parklawn Computer Center is a US Government Computer Center for the Federal Food and Drug Administration. Verify here.

Tuesday, May 01, 2007
Monogram Bioscience stock drops 5%
Early selling in conjunction with the revelation on this site that the Office of Inspector General at the Department of Health and Human Services has contacted the Pfizer HIV whistleblower initially pushed the stock down 5%. Monogram is right now trading about 2% below yesterday's price, when it dropped about 4%, for a total loss in two days of 6%.
During yesterday and today, the stock has gone from a high of $1.97 to a low of $1.76 and is now trading at $1.83.
Also see my original story "Pfizer whistleblower accuses company of using sales force to illegally market new AIDS-drug before FDA approval"
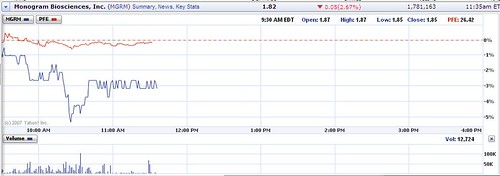
Monogram Bioscience now trades about 6% below the price before the disclosures were made on this blog and 16% below the price 5 days ago.

During yesterday and today, the stock has gone from a high of $1.97 to a low of $1.76 and is now trading at $1.83.
Also see my original story "Pfizer whistleblower accuses company of using sales force to illegally market new AIDS-drug before FDA approval"

Monogram Bioscience now trades about 6% below the price before the disclosures were made on this blog and 16% below the price 5 days ago.

OIG REQUESTS MEETING WITH PFIZER HIV WHISTLEBLOWER.
The Pfizer whistleblower in my story "Pfizer whistleblower accuses company of using sales force to illegally market new AIDS-drug before FDA approval" has contacted the Office of Inspector General at the Department of Health and Human Services with her concerns about what she alleges is Pfizer's illegal premarketing of the HIV/AIDS drug maraviroc. The Pfizer whistleblower just forwarded the response she received from an agent at the OIG:
I just wanted to send you a quick note to let you know that I have
received your emails. I am still in trial, but I do want to meet with
you in the next week or two. I will contact you (probably later next
week) to set up a time to meet up. If you have any additional
informaiton, feel free to send it along.
Thanks!
Special Agent NAME REDACTED
U.S. Department of Health and Human Services
Office of Inspector General
50 Kennedy Plaza, Suite 750
Providence, RI 02903
Phone: REDACTED
Fax: REDACTED
One reason the OIG is interested in this situation is that Pfizer has been forced to sign not just one, but two corporate integrity agreements (CIA) with this agency (download here), promising to conduct their business in an ethical manner.
Pfizer is obligated to notify the OIG of all "reportable events" within 30 days, such as suspicions of any illegal behavior. If Pfizer would not do this they would be in violation of the terms of the CIA and could receive fines, or, at worst, be excluded from federal health care programs.
I just wanted to send you a quick note to let you know that I have
received your emails. I am still in trial, but I do want to meet with
you in the next week or two. I will contact you (probably later next
week) to set up a time to meet up. If you have any additional
informaiton, feel free to send it along.
Thanks!
Special Agent NAME REDACTED
U.S. Department of Health and Human Services
Office of Inspector General
50 Kennedy Plaza, Suite 750
Providence, RI 02903
Phone: REDACTED
Fax: REDACTED
One reason the OIG is interested in this situation is that Pfizer has been forced to sign not just one, but two corporate integrity agreements (CIA) with this agency (download here), promising to conduct their business in an ethical manner.
Pfizer is obligated to notify the OIG of all "reportable events" within 30 days, such as suspicions of any illegal behavior. If Pfizer would not do this they would be in violation of the terms of the CIA and could receive fines, or, at worst, be excluded from federal health care programs.
Message from a Monogram Bioscience investor.
My story yesterday "Pfizer whistleblower accuses company of using sales force to illegally market new AIDS-drug before FDA approval" received quite a bit of attention from Monogram Bioscience investors. They seem to fear what the fall-out may do to Monogram stock.
The biotech company has its fortunes closely aligned with Pfizer and the success of the drug I wrote about, maraviroc. Monogram Biosciences makes a blood test to determine if a patient falls into the category that should be treated with the Pfizer drug.
 Yesterday Monogram stock fell about 4%, but the Monogram shares have experienced downward pressure since last week.
Yesterday Monogram stock fell about 4%, but the Monogram shares have experienced downward pressure since last week.
It doesn't make any difference to me, since I don't hold any stock but it certainly makes a difference to Monogram investors.
And I like to present a balanced picture.
An opportunity to do just that arose when a Monogram investor wrote to me yesterday and said, "Your National Inquirer approach is disgusting and deplorable! I understand you are disgruntled and you have an axe to grind, but in the process you are screwing a lot of other people."
So I invited him to write something I could use, from his perspective. This is his response:
"----Original Message Follows----
From: redacted@aol.com\
To: rostpeter@hotmail.com
Subject: Re: PFE article
Date: Mon, 30 Apr 2007 22:32:16 EDT
Peter,
I don't want you to write anything. I think the whole story is ridiculous and false. If this person wanted to remain anonymous, "she" certainly wouldn't be anymore based on what was written in that "statement." Also, on your blog site you write that some law firm called you ready to represent you... why in the world would you need representation if you are telling the truth. You also elude to a network picking up the article, but then go on to hint at the possibility that it wouldn't happen because the "whistle blower" may not be comfortable coming forward. The description of the "WB" and her position, and the meetings she attended and with whom would make it clear to anyone on the inside who the "WB" is.
My concern with this article/story is that I am an investor in another company that is in collaboration with Pfizer, and I am sure that along with myself there are plenty of others that are investing in their children's futures via the stock market. I know how sometimes the least little thing can effect small bio-tech's while this story won't have any effect whatsoever on Pfizer. I understand you are disgruntled, but don't drag others into your frustration with the company. Sometimes what you print has far-reaching effects beyond your intended target. If this is true, then collect far more information and tie up the many loose ends and then print the story with names, incidences and real substance. I am a journalism and mass comm. major, and if I would have brought this story to my producer, I would get laughed at thoroughly. This is irresponsible on your part, in my opinion, and as long as it's missing the many facts that it needs to not scream of libelous journalism, it's not ready for print.
Just my opinion,
NAME REDACTED"
Perhaps a couple of clarifications are needed. The law firm didn't ask to represent me . . . they asked to represent the Pfizer whistleblower. And there were many persons who could have given me the information I published yesterday. There are quite a few Pfizer employees less than pleased with Pfizer's response to ethical issues.
As for the Monogram investor, this was my response:
"Thanks for this response. I'm sorry to tell you this may get worse, real fast. You may want to rebalance your portfolio. Stay tuned for update today.
P."
The biotech company has its fortunes closely aligned with Pfizer and the success of the drug I wrote about, maraviroc. Monogram Biosciences makes a blood test to determine if a patient falls into the category that should be treated with the Pfizer drug.
 Yesterday Monogram stock fell about 4%, but the Monogram shares have experienced downward pressure since last week.
Yesterday Monogram stock fell about 4%, but the Monogram shares have experienced downward pressure since last week.It doesn't make any difference to me, since I don't hold any stock but it certainly makes a difference to Monogram investors.
And I like to present a balanced picture.
An opportunity to do just that arose when a Monogram investor wrote to me yesterday and said, "Your National Inquirer approach is disgusting and deplorable! I understand you are disgruntled and you have an axe to grind, but in the process you are screwing a lot of other people."
So I invited him to write something I could use, from his perspective. This is his response:
"----Original Message Follows----
From: redacted@aol.com\
To: rostpeter@hotmail.com
Subject: Re: PFE article
Date: Mon, 30 Apr 2007 22:32:16 EDT
Peter,
I don't want you to write anything. I think the whole story is ridiculous and false. If this person wanted to remain anonymous, "she" certainly wouldn't be anymore based on what was written in that "statement." Also, on your blog site you write that some law firm called you ready to represent you... why in the world would you need representation if you are telling the truth. You also elude to a network picking up the article, but then go on to hint at the possibility that it wouldn't happen because the "whistle blower" may not be comfortable coming forward. The description of the "WB" and her position, and the meetings she attended and with whom would make it clear to anyone on the inside who the "WB" is.
My concern with this article/story is that I am an investor in another company that is in collaboration with Pfizer, and I am sure that along with myself there are plenty of others that are investing in their children's futures via the stock market. I know how sometimes the least little thing can effect small bio-tech's while this story won't have any effect whatsoever on Pfizer. I understand you are disgruntled, but don't drag others into your frustration with the company. Sometimes what you print has far-reaching effects beyond your intended target. If this is true, then collect far more information and tie up the many loose ends and then print the story with names, incidences and real substance. I am a journalism and mass comm. major, and if I would have brought this story to my producer, I would get laughed at thoroughly. This is irresponsible on your part, in my opinion, and as long as it's missing the many facts that it needs to not scream of libelous journalism, it's not ready for print.
Just my opinion,
NAME REDACTED"
Perhaps a couple of clarifications are needed. The law firm didn't ask to represent me . . . they asked to represent the Pfizer whistleblower. And there were many persons who could have given me the information I published yesterday. There are quite a few Pfizer employees less than pleased with Pfizer's response to ethical issues.
As for the Monogram investor, this was my response:
"Thanks for this response. I'm sorry to tell you this may get worse, real fast. You may want to rebalance your portfolio. Stay tuned for update today.
P."
Monday, April 30, 2007
Monogram Bioscience investor comments on allegations of illegal Pfizer HIV premarketing: "I think we're screwed"
Pfizer is seeking FDA approval to sell maraviroc for patients who have tried other treatments and have a form of HIV that uses the CCR5 receptor. Today, Question Authority revealed that a Pfizer whistleblower claims Pfizer illegally premarketed maraviroc.
This didn't sit well with Monogram Biosciences investors. Monogram Biosciences makes a blood test to determine if a patient falls into the category that should be treated with the Pfizer drug. In Pfizer's screening, 56 percent of previously treated patients had a virus that used CCR5.
Here are a few comments about my article, from people with an apparent stake in Monogram Biosciences, posted on the company's financial message board:
"I dont think its a real setback. If they broke the "Marketing" rules then PFE will pay a fine and they'll move on. This has nothing to do with the success, effectiveness or approval of the drug. I doubt that the FDA will sacrafice human lives for the sake of "Marketing". I think this "illegal marketing" reinforces PFE's confidence in this drug."
"did pfe respond to this yet? "
"I think you are smart enough not to believe everything that you see printed or in the net."
"I know. I try to post my finds though, so people can make an informed decision. I hold a very large position in this stock and that article doesn't alter my particular views."
"I agree, there is a lot of BS out there... seems this guy who wrote the article (Dr. Rost) has a personal vendetta against PFE. He has a website where all he does is bash PFE... I just read that he has already retained the services of Katz, Marshall & Banks, LLP to represent this supposed whistleblower. I'm thinking this is much to do about nothing. http://peterrost.blogspot.com/ "
"i think where screwed"
This didn't sit well with Monogram Biosciences investors. Monogram Biosciences makes a blood test to determine if a patient falls into the category that should be treated with the Pfizer drug. In Pfizer's screening, 56 percent of previously treated patients had a virus that used CCR5.
Here are a few comments about my article, from people with an apparent stake in Monogram Biosciences, posted on the company's financial message board:
"I dont think its a real setback. If they broke the "Marketing" rules then PFE will pay a fine and they'll move on. This has nothing to do with the success, effectiveness or approval of the drug. I doubt that the FDA will sacrafice human lives for the sake of "Marketing". I think this "illegal marketing" reinforces PFE's confidence in this drug."
"did pfe respond to this yet? "
"I think you are smart enough not to believe everything that you see printed or in the net."
"I know. I try to post my finds though, so people can make an informed decision. I hold a very large position in this stock and that article doesn't alter my particular views."
"I agree, there is a lot of BS out there... seems this guy who wrote the article (Dr. Rost) has a personal vendetta against PFE. He has a website where all he does is bash PFE... I just read that he has already retained the services of Katz, Marshall & Banks, LLP to represent this supposed whistleblower. I'm thinking this is much to do about nothing. http://peterrost.blogspot.com/ "
"i think where screwed"
Press about Pfizer's HIV whistleblower affair.
Pharmalot: Did Pfizer Improperly Sell An HIV Drug?
PharmaGossip: Pfizer - maraviroc: whoops there goes another Corporate Integrity Agreement
Brandweek: It's Already a Bad Day at Pfizer, as PR Problems Arise on Lipitor, Maraviroc and Viagra
Bill of Rights: Pfizer whistleblower accuses company of using sales force to illegally market new AIDS-drug before FDA approval. And Promotion and charging for investigational drugs.
The Black Kitty: Pfizer's First Female Whistleblower: Illegal Marketing of HIV Drug
And . . . one of the big television networks just contacted me . . . but I'm not sure Pfizer's whistleblower is ready to go live, just yet.
PharmaGossip: Pfizer - maraviroc: whoops there goes another Corporate Integrity Agreement
Brandweek: It's Already a Bad Day at Pfizer, as PR Problems Arise on Lipitor, Maraviroc and Viagra
Bill of Rights: Pfizer whistleblower accuses company of using sales force to illegally market new AIDS-drug before FDA approval. And Promotion and charging for investigational drugs.
The Black Kitty: Pfizer's First Female Whistleblower: Illegal Marketing of HIV Drug
And . . . one of the big television networks just contacted me . . . but I'm not sure Pfizer's whistleblower is ready to go live, just yet.
Eminent Washington D.C. law firm offers to represent Pfizer whistleblower
The law firm Katz, Marshall & Banks, LLP, has just contacted me and offered to assist the Pfizer whistleblower in my story "Pfizer whistleblower accuses company of using sales force to illegally market new AIDS-drug before FDA approval."
David Marshall writes, "I just read your very interesting article on OpEdNews.com about Pfizer whistleblower Jane Roe, who has aired allegations of unlawful marketing of maraviroc. My law partner, Debra Katz, and I have successfully represented a number of whistleblowers in the pharmaceutical and medical industries (see our web site), and have focused on the very issues that Ms. Roe has raised. We would be glad to speak with Ms. Roe if she does not yet heave a lawyer. Please let her know that we are available if she needs representation."
Katz, Marshall & Banks, LLP, is dedicated to working for social justice by providing high-quality legal representation to individuals, groups and causes that are under-served in our legal system. They are dedicated to using our legal expertise and skills to support the civil rights, increase access to legal services, and obtain equitable and just treatment for employees and consumers. When other avenues of redress have been exhausted, they are the ones who take whatever legal action is appropriate to challenge governmental, corporate and individual wrongdoing, and to win compensation and vindication for those who have been harmed.
Sounds pretty good to me.
Perhaps YOU should contact Katz, Marshall & Banks, LLP?
More info here.
David Marshall writes, "I just read your very interesting article on OpEdNews.com about Pfizer whistleblower Jane Roe, who has aired allegations of unlawful marketing of maraviroc. My law partner, Debra Katz, and I have successfully represented a number of whistleblowers in the pharmaceutical and medical industries (see our web site), and have focused on the very issues that Ms. Roe has raised. We would be glad to speak with Ms. Roe if she does not yet heave a lawyer. Please let her know that we are available if she needs representation."
Katz, Marshall & Banks, LLP, is dedicated to working for social justice by providing high-quality legal representation to individuals, groups and causes that are under-served in our legal system. They are dedicated to using our legal expertise and skills to support the civil rights, increase access to legal services, and obtain equitable and just treatment for employees and consumers. When other avenues of redress have been exhausted, they are the ones who take whatever legal action is appropriate to challenge governmental, corporate and individual wrongdoing, and to win compensation and vindication for those who have been harmed.
Sounds pretty good to me.
Perhaps YOU should contact Katz, Marshall & Banks, LLP?
More info here.
Subscribe to:
Posts (Atom)